The study investigates how post-gelation CO₂ release rates affect hydrogel properties, informing medical application development
Hydrogels created using carbon dioxide (CO₂) offer a safer alternative to those formed with acidic agents. While most research has focused on pre-gelation conditions affecting hydrogel properties, this study by researchers from Tokyo University of Science explores the impact of CO₂ release after gelation. The team prepared alginate-based hydrogels and found that faster CO₂ release decreases crosslinking, while slower release results in stiffer hydrogels. These findings could lead to improved hydrogels for medical applications.
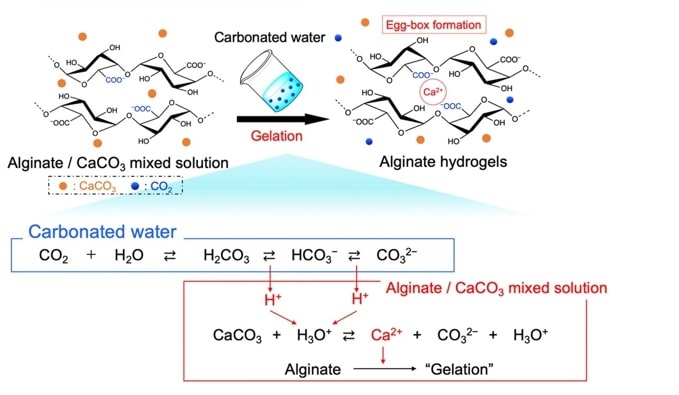
Hydrogels, which are soft materials made of water-filled, crosslinked polymer networks, have a wide range of uses, from wound dressings to enhancing soil moisture for plant growth. They are formed through a process called gelation, where polymers in a solution are linked together to form a gel. Biopolymers, such as polysaccharides and proteins, often require the addition of acidic agents for this gelation process. However, these agents can remain in the hydrogel, posing risks for biological applications. To address this issue, a new gelation method uses carbon dioxide (CO₂) instead of acidic agents. CO₂ acts as an acidic agent during gelation but escapes into the atmosphere once the hydrogel forms.
A study led by Professor Hidenori Otsuka and Mr. Ryota Teshima from Tokyo University of Science, Japan, investigated the effect of CO2 release on the properties of hydrogels. Their findings were made available online on June 5, 2024, were published in Issue 16 of the journal Materials Advances on August 21, 2024 and provide valuable insights for synthesizing hydrogels suitable for medical uses.
“The degree of crosslinking in hydrogels is typically controlled by ‘pre-gelation parameters,’ such as polymer and crosslinker concentrations. However, we demonstrate that the crosslinking degree of hydrogels prepared using carbon dioxide as the acidic agent is also influenced by post-gelation conditions,” says Prof. Otsuka and Mr. Teshima.
The researchers synthesized hydrogels called Alg-gels from alginate, a polymer derived from brown seaweed. They mixed alginate with calcium carbonate (CaCO₃) and added carbonated water, resulting in a porous hydrogel where alginate chains were crosslinked by calcium ions. To control the release of CO₂ from the Alg-gels, two samples were prepared: one incubated in a Petri dish, exposing only its top surface to air, and another on a wire mesh, exposing the entire surface. The rate of CO₂ release was monitored using bromothymol blue (BTB), a pH indicator that changes color with acidity (yellow in acidic conditions, green when neutral, and blue in alkaline conditions).
The gel in the Petri dish gradually turned green (neutral) over 60 minutes, indicating slow CO₂ release, and became fully blue after 5 hours. In contrast, the gel on the wire mesh released CO₂ much faster, turning fully blue by 40 minutes and releasing all the CO₂ in just 90 minutes.
The rapid release of CO₂ immediately after gel formation prevented the calcium carbonate from fully dissolving in the solution, leaving fewer calcium ions available to link the polymer chains. “Rapid release of CO2 from the hydrogel after gelation increased the pH of the system and decreased the degree of crosslinking,” explain Prof. Otsuka and Mr. Teshima. When both samples were subjected to a compression test, the stiffness, breaking stress, and energy required to break the disks were higher for those incubated in the petri dish compared to those on the wire mesh.
This study enhances our understanding of how CO₂ release after gel formation affects the degree of crosslinking and the mechanical properties of hydrogels, providing insights for creating hydrogels using CO₂. Additionally, the use of alginic acid derived from marine waste can transform waste into high-value hydrogels for medical uses such as tissue engineering, including wound healing and organ regeneration.
***
Reference
Title of original paper: Effect of CO2 release behavior on the crosslinking degree of alginate hydrogels prepared with CaCO3 and carbonated water
Journal: Materials Advances
DOI: 10.1039/D4MA00257A

Leave a Reply